Biosimilarity FDA perspective バイオシミラー
(税込) 送料込み
商品の説明
定価 約27,、現在アマゾンの最安値が約17,です。
バイオシミラー薬・FDAについての洋書のハードカバー本です。
●状態:表紙の角がやや擦れていますが、中身は一部のみ読んだほぼ新品です。
●ヤケ:なし
●折り目:なし
●書き込み:なし
●その他、注意事項:
中古品ということをご理解の上ご検討ください。宜しくお願いします商品の情報
| カテゴリー | 本・音楽・ゲーム > 本 > 洋書 |
|---|---|
| 商品の状態 | 未使用に近い |

宅配便配送 Biosimilarity FDA perspective バイオシミラー 洋書

Biosimilarity: The FDA Perspective
休日 Biosimilarity FDA perspective バイオシミラー savingssafari.com

Biosimilarity: The FDA Perspective See more 1st Edition1st Edition

休日 Biosimilarity FDA perspective バイオシミラー savingssafari.com

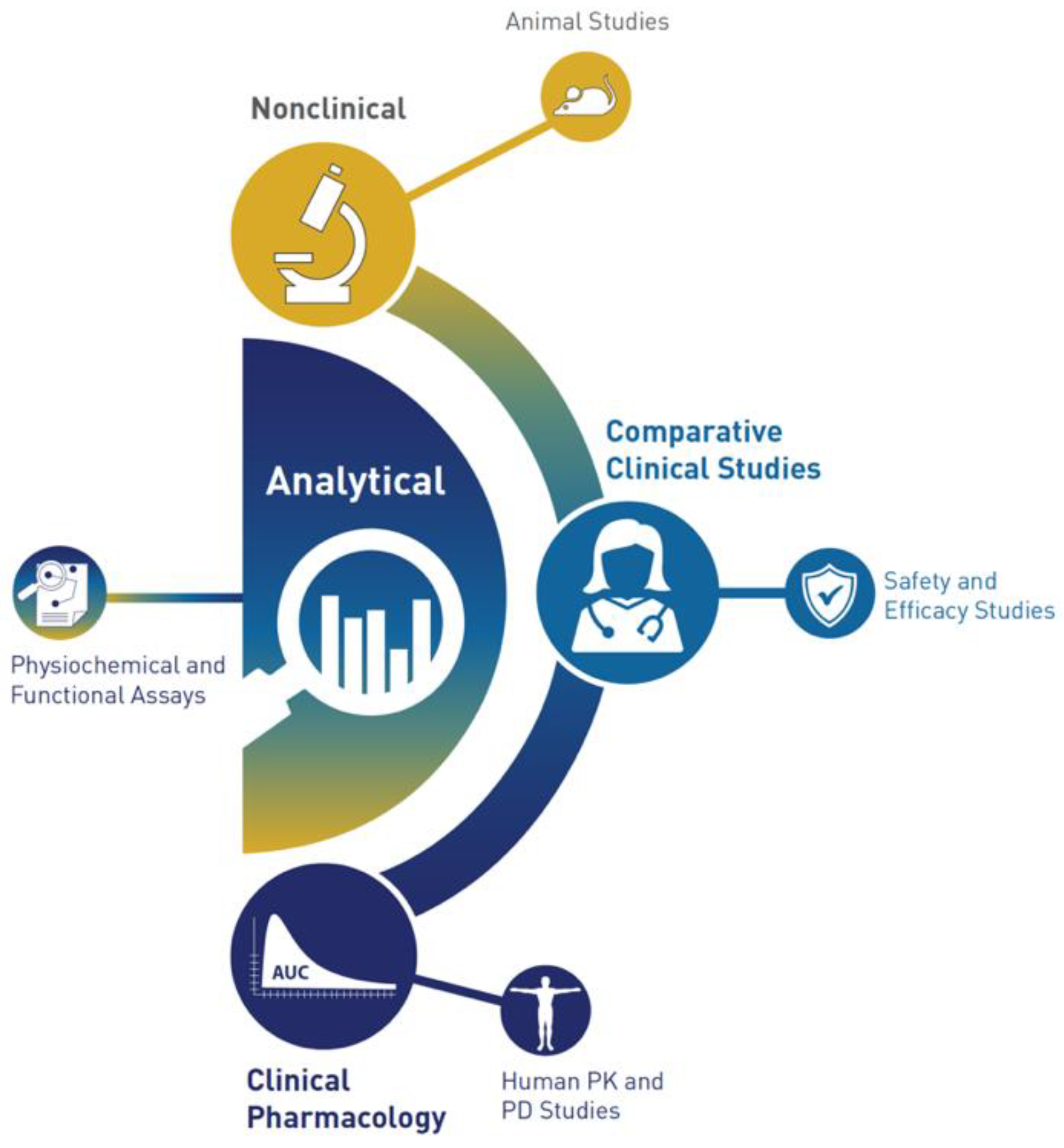
An FDA perspective on the assessment of proposed biosimilar
休日 Biosimilarity FDA perspective バイオシミラー savingssafari.com

宅配便配送 Biosimilarity FDA perspective バイオシミラー 洋書

宅配便配送 Biosimilarity FDA perspective バイオシミラー 洋書

休日 Biosimilarity FDA perspective バイオシミラー savingssafari.com

Novartis, Roche, and Pfizer respond to FDA Biosimilar Action Plan

The Top 5 Biosimilar Articles for the Week of June 26
宅配便配送 Biosimilarity FDA perspective バイオシミラー 洋書
The Biosimilar Market: What You Need to Know - Retina Today

Biosimilar Clinical Trials and US FDA Guidance | ProRelix Research

Pharmaceutics | Free Full-Text | The Biosimilar Landscape: An
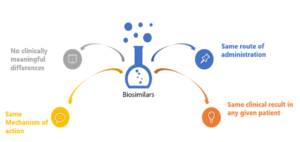
The Journey from Biologic to Biosimilar—A Clinical Perspective - ACRP

The Basics of Biosimilars

Pharmacodynamic Biomarkers: Their Role in Biosimilar Product
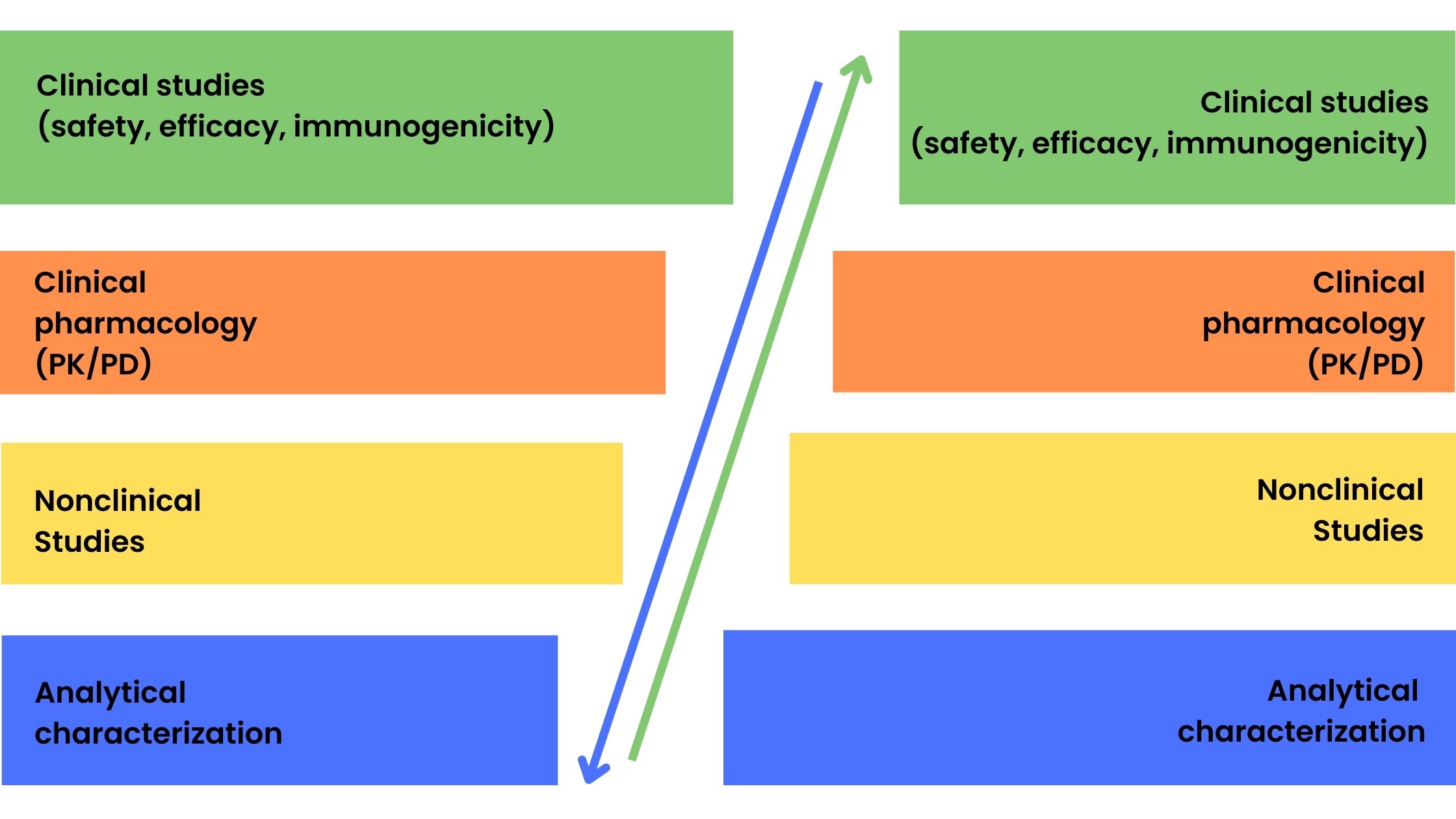
Biosimilar Clinical Trials and US FDA Guidance | ProRelix Research

Provider and Patient Knowledge Gaps on Biosimilars: Insights From

Biosimilar Clinical Trials and US FDA Guidance by ProRelix Info

FDA Perspectives on Biosimilar BLA-Manufacturing (28of33) Quality – Oct. 16-17, 2019

Tentative Biosimilar Approvals A Consideration for the US FDA
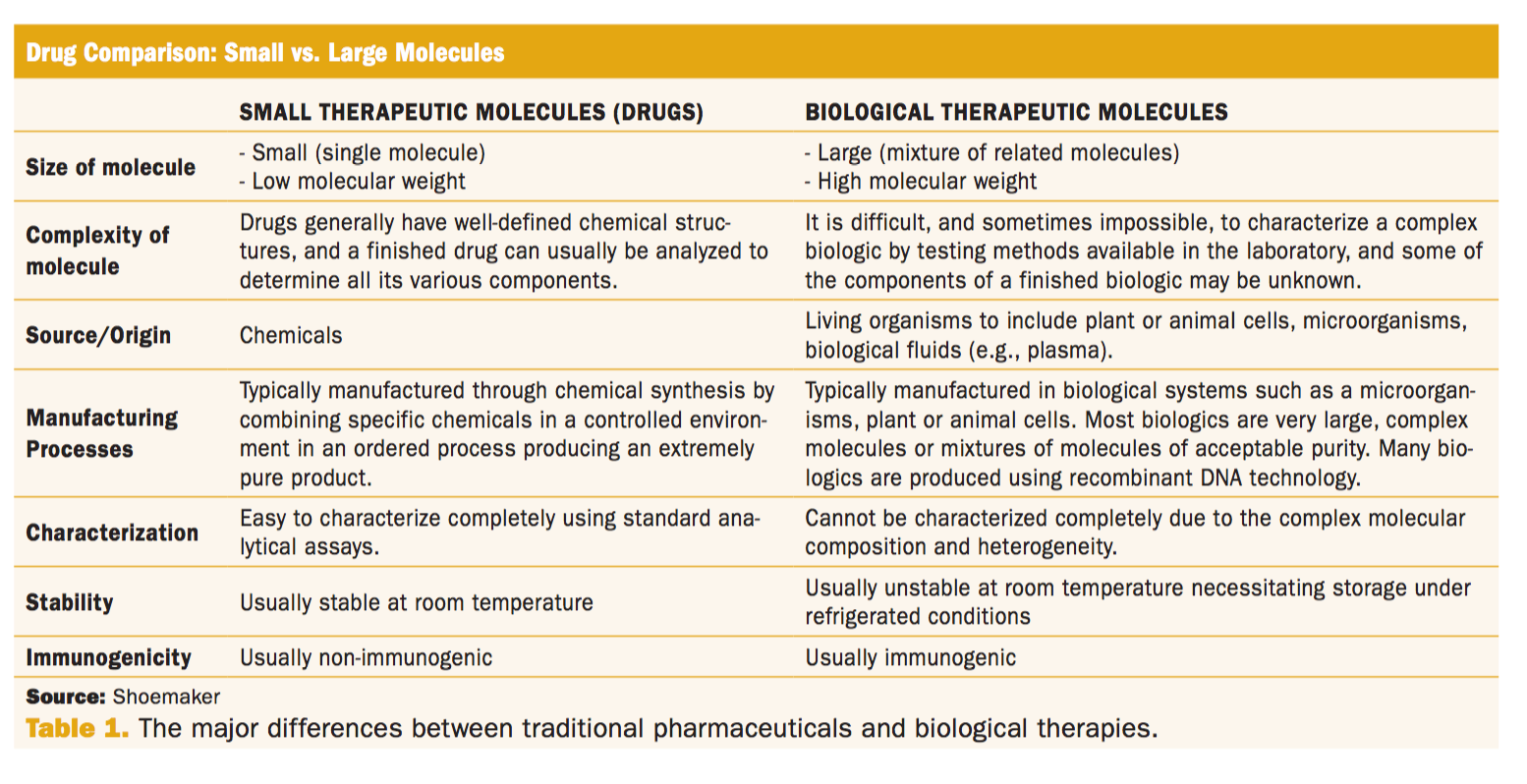
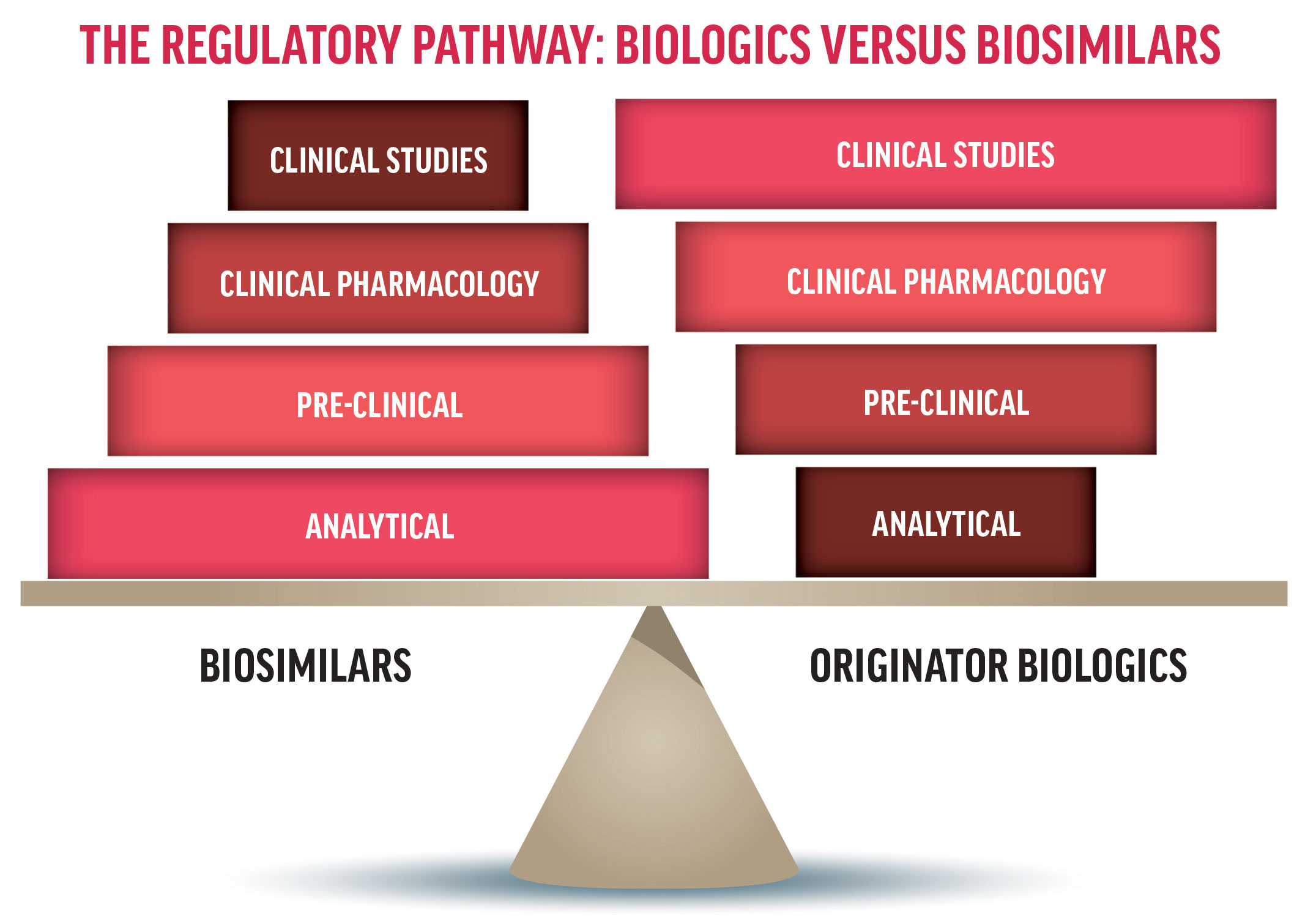
The U.S. Biosimilar Pathway: Policy Precedes Science
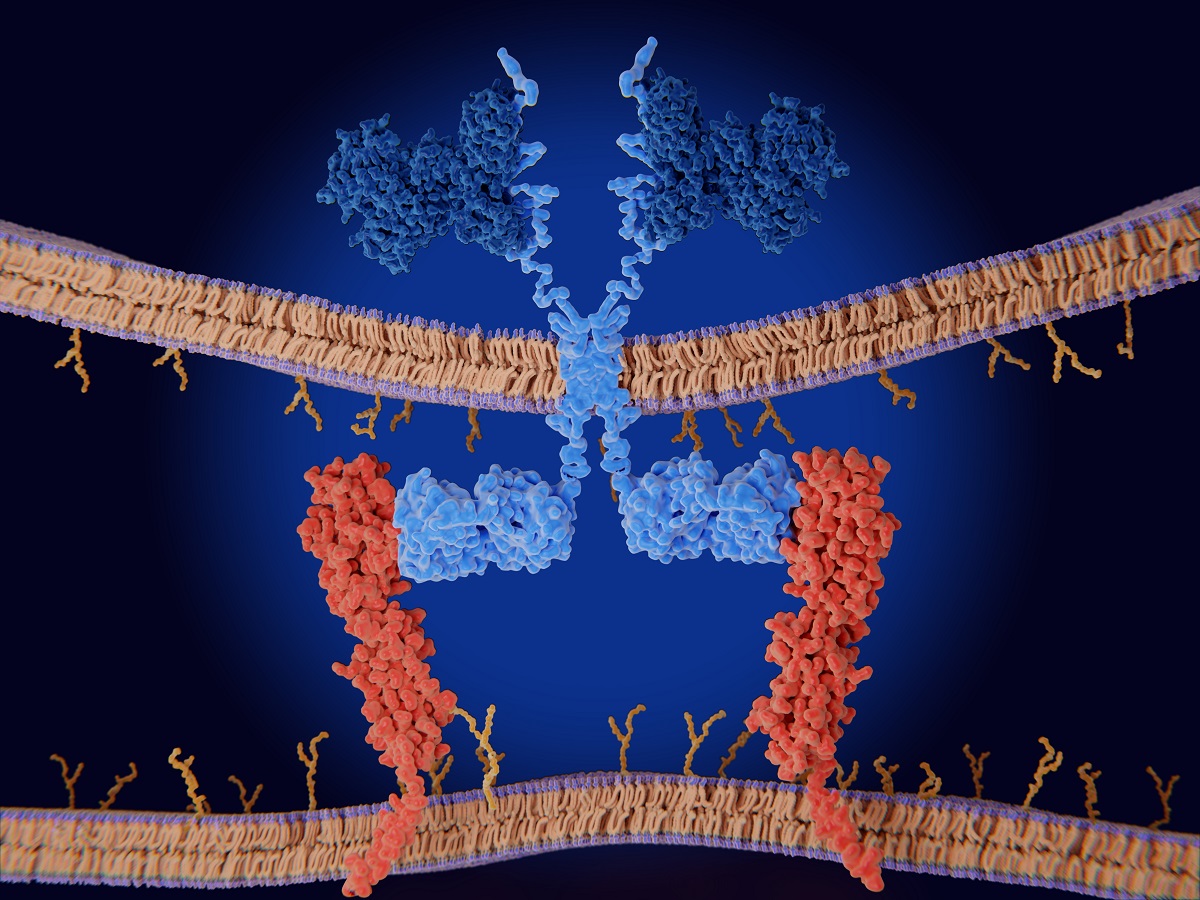
Functional Biosimilarity to Replace Clinical Efficacy Testing of
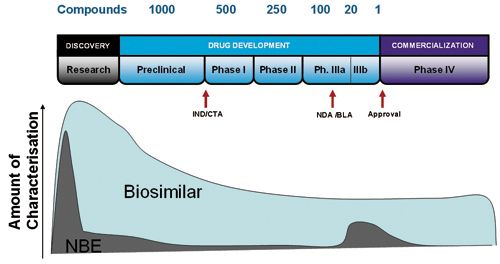
Key Considerations in Biosimilars Development

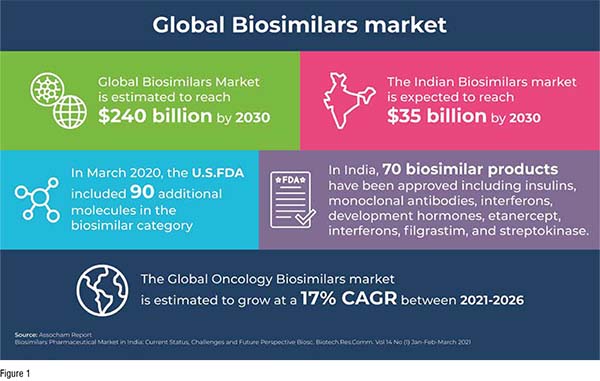
Biosimilars in Developed Economies: Overview, Status, and

FDA approval of biosimilars in the United States | Download Table
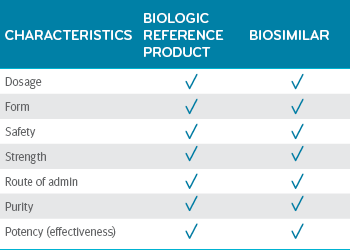
Biosimilars are here to help, really - Prime Therapeutics LLC

Biosimilars
Biosimilars 2022 Year in Review
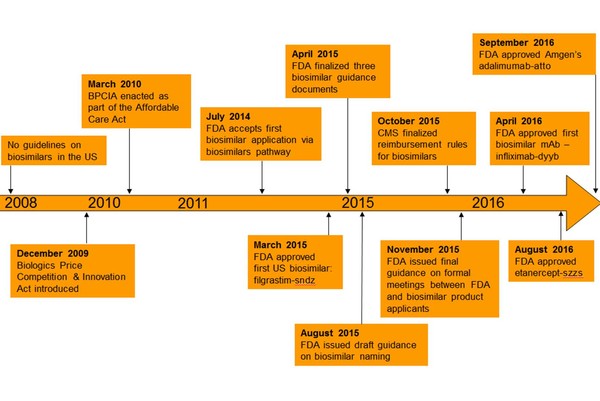
The evolution of biosimilars in the US

FDA Releases Biosimilars Guidelines to Little Fanfare

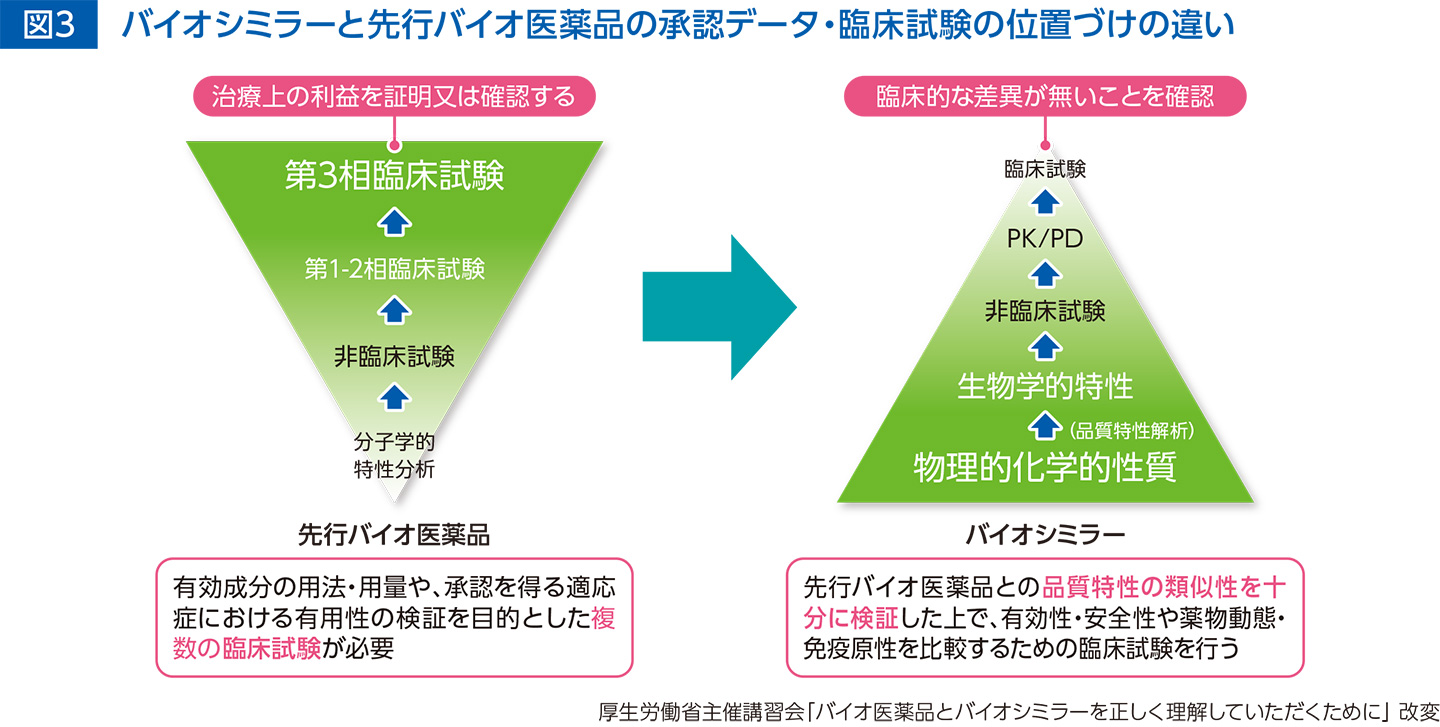
Biosimilar or Not: Physicochemical and Biological Characterization

Revisiting Interchangeability to Realize the Benefit of Biosimilars

Safety, Immunogenicity and Interchangeability of Biosimilar

Biosimilarity assessment of biosimilar therapeutic monoclonal

From our perspective: Biosimilar product labeling | FDA
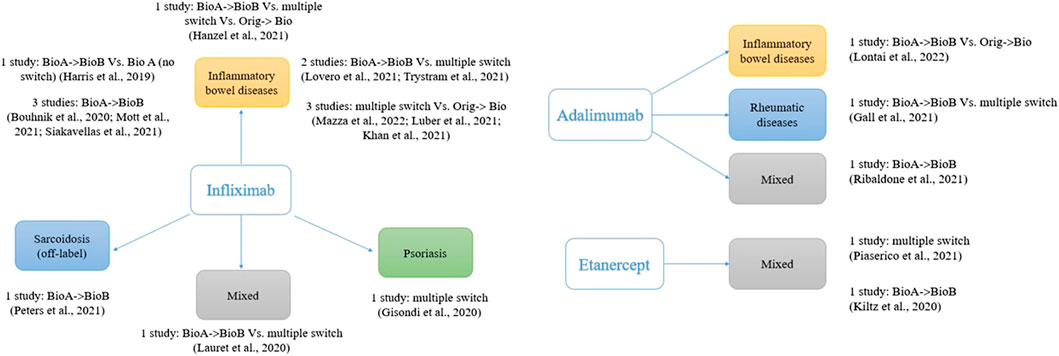
Frontiers | Switching Among Biosimilars: A Review of Clinical Evidence


商品の情報
メルカリ安心への取り組み
お金は事務局に支払われ、評価後に振り込まれます
出品者
スピード発送
この出品者は平均24時間以内に発送しています














